SIMIT made a progress in the microstructure investigation on hexagonal boron nitride
Date:01-07-2019 | 【Print】 【close】
Two dimensional (2D) hexagonal boron nitride (h-BN) is an extraordinary mesh consisting of alternating sp2-bonded boron and nitrogen atoms. As the only known wide bandgap insulator in the family of 2D layered materials, h-BN is acknowledged to be an ideal substrate and tunneling barrier for electronic devices. Moreover, the exceptional thermal and chemical stability of h-BN enables it to function as an anti-oxidation layer, even at temperatures as high as 1100 °C. This makes h-BN a suitable choice for ultrathin anti-oxidization coatings. In addition, another key feature of h-BN is its impermeability to almost all common gases, but transparency to protons. This enables h-BN to act as a selective membrane to extract and store hydrogen from hydrocarbon or mixed-gas plasmas.
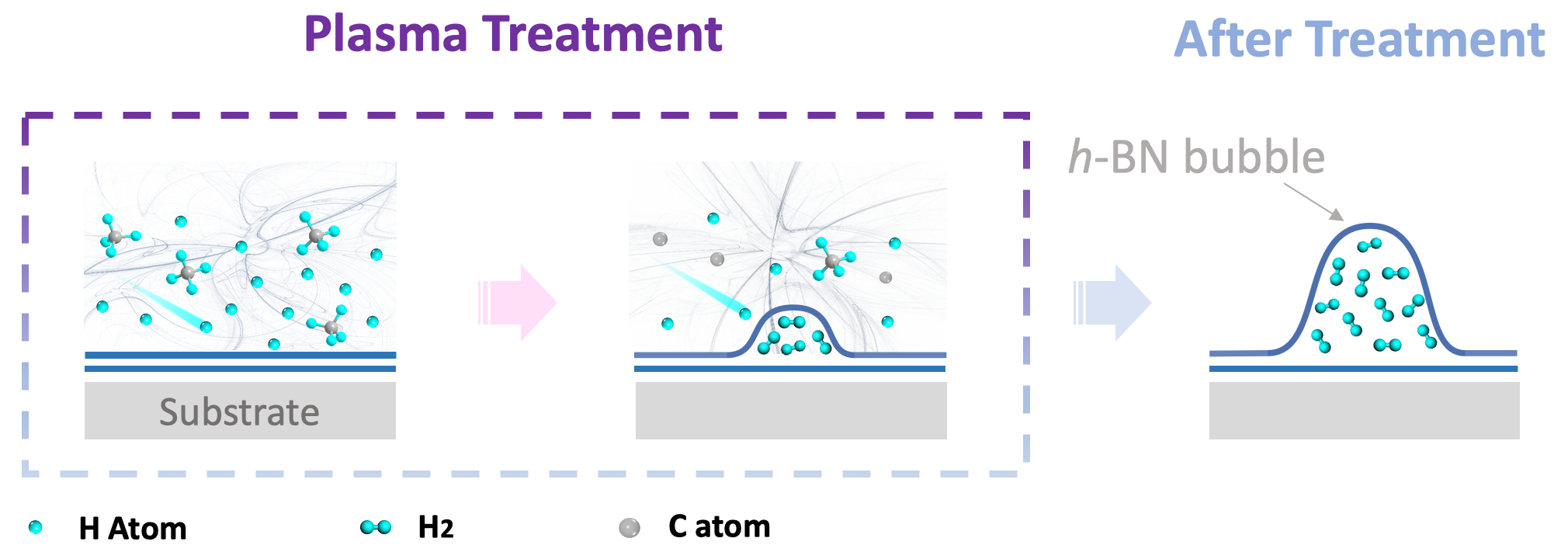
Fig. 1 | Extracting hydrogen from hydrocarbon gases by a plasma treatment.
To make full use of these special properties of h-BN, scientists from Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences carried out plasma treatment with hydrogen, methane or acetylene on h-BN multilayers . They found that all these hydrogen-containing plasmas can trap hydrogen gas into the h-BN interlayers exhibited by the formation of bubbles on its surface. (see in Fig. 1)
A detailed STEM investigation on h-BN is helpful to unveil the reason why protons can easily penetrate the mesh without barrier. Different to graphite, whose layers are usually staggered by an AB stacking sequence, multilayered h-BN exhibits excellent proton conductivity due to its favoring the porous stacking structure. The aligned pores in the out-of-plane direction allow protons to move unimpededly throughout the space. This characteristic structure of multilayered h-BN could facilitate its use in hydrogen storage applications.
A low-temperature atomic force microscope system allows researchers to prove that h-BN bubbles are indeed filled with hydrogen gas. The sample chamber for AFM measurement was filled with 4He in a pressure of 5 mbar for heat-exchange with temperature controllable from 4 to 300K.
Topography of the same bubbles was measured at temperature from 30 to 38K. It is found that the bubbles deflate at 33K while they are inflated with gases at 34K. The deflating/swelling processes are reproducible via cooling/heating the sample. The highest temperature at which the bubbles become flat is recognized as the liquid-vapor phase transition temperature (Ttransition), which exhibits an average value equal to 33.2±3.9 K. This value is very close to Ttransition of H2 (33.18 K) among all possible gases.
For more details, please see the paper: “Isolating hydrogen in hexagonal boron nitride bubbles by a plasma treatment” published in Nature Communications. (https://doi.org/10.1038/s41467-019-10660-9)